ORIGINAL ARTICLE | https://doi.org/10.5005/jp-journals-10054-0109 |
Association of Age and Ethnicity with Alcoholic Liver Diseases in East Sikkim
1–4Department of Biochemistry, Sikkim Manipal Institute of Medical Sciences, Gangtok, Sikkim, India
Corresponding Author: Sonam C Bhutia, Department of Biochemistry, Sikkim Manipal Institute of Medical Sciences, Gangtok, Sikkim, India, Phone: +91 9735974048, e-mail: namsokagalpa@gmail.com
How to cite this article Rai S, Bhutia SC, Dhakal S, et al. Association of Age and Ethnicity with Alcoholic Liver Diseases in East Sikkim. Indian J Med Biochem 2019;23(3):309–311.
Source of support: Nil
Conflict of interest: None
ABSTRACT
Background: Alcoholic liver disease (ALD) is most prevalent in Sikkim where its consumption is a common practice and accepted socially.
Materials and methods: Fifty male patients and normal healthy groups were taken for the study. Direct and total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase (ALP), and reduced glutathione along with the relationship with age group and ethnicity were assessed.
Results and conclusion: Biochemical parameters such as direct (1.6 ± 1.0) and total bilirubin (2.9 ± 1.5), aspartate aminotransferase (68.7 ± 1.629), alanine aminotransferase (29.8 ± 6.5), ALP (1.4 ± 3.4), gamma-glutamyl transferase (1.18 ± 8.4) were observed to be increased and oxidative stress markers like glutathione was decreased in patients with alcoholic liver disease. Highest number of patients with the disease were between 36 and 46 years (44%) of age and in Kiratis (40%). This is a first attempt to study the ALD based on age group and ethnicity. Various programs should initiated to prevent alcohol abuse.
Keywords: Alcoholic liver disease, Alkaline phosphatase, Ethnicity, Glutathione.
INTRODUCTION
Alcohol consumption is a worldwide problem and the burden of the disease remains significant in most countries among which ALD has the highest mortality rate.1 It represents a wide range of clinical illness and morphological changes including from fatty liver to hepatic inflammation and alcoholic hepatitis to alcoholic cirrhosis.2
According to the World Health Organization (WHO), alcohol-related diseases are the third cause of death and disability in most developed countries.3 The National Institute on Alcohol Abuse and Alcoholism recommends that both males and females should not drink more than 28 g and 14 g per day, respectively.4
Alcohol use has become an important public health issue in the small Himalayan state of Sikkim. According to the National Family Health Survey-4, Government of India, it has highlighted a significant prevalence of alcohol use in the state: 51.2% and 23% among above 15–49 years of age in males and females, respectively.5 12% of deaths who were medically certified are due to alcohol abuse recorded from an unpublished data which is alarming.
Oxidative stress has been associated with numerous factors involved in the progression of alcohol-induced liver injury. Glutathione is the most abundant redox molecules in the cells and thus the most important in determining the status of cellular redox.6 Conventional biochemical markers such as alanine transaminase (ALT), aspartate transaminase (AST), ALP, and gamma glutamyl transferase (GGT) are used to screen the status and reflect any injury of the liver.
Alcohol consumption is the most common practice in Sikkim leading to various health hazards; hence, we aim to evaluate the biochemical parameters among the patients with ALD and healthy volunteers and to find an association based on age group and ethnicity.
MATERIALS AND METHODS
Study Design
It was a cross-sectional study conducted in the Department of Biochemistry, Sikkim Manipal Institute of Medical Sciences, Gangtok, Sikkim and the duration of the study was from July 2016 to September 2016. Written informed consent was obtained from each participant. The patients with ALD were diagnosed by the consultant from the Department of Medicine, Gangtok, Sikkim.
The sample size was calculated by using the following formula (Sathian, et al.):7
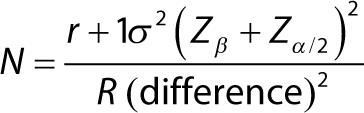
A total of 50 patients and 50 healthy volunteers were taken.
Inclusion Criteria
Fifty male patients diagnosed with ALDs, age above 25 years, and 50 healthy volunteers with age and sex matched. Only male patients were taken, because as per the NFHS-4, the significant prevalence of alcohol use in males is higher than females.5
Sample Processing
5 mL of venous whole blood was collected in a plain and ethylenediaminetetraacetic acid vial. The serum was obtained by centrifugation (3,000 rpm) and used for the estimation of following parameters:
- Total and direct bilirubin (TB and DB) (Jendrassik and Grof)8
- Aspartate amino transferase (AST) (Thefelt et al.)9
- Alanine transferase (ALT) (Bergmeyer et al.)10
- Alkaline phosphatase (ALP) (Bergmeyer et al.)11
- Gamma glutamyl transferase (GGT) (Szasz)12
The other part of the whole blood was used for the estimation of reduced glutathione (GSH) (Beutler et al.).13 The sample was analyzed in spectrophotometer (Erba – Chem 5 plus V2).
Statistical analysis was done using the Statistical Package for the Social Sciences version 16.0 (SPSS Inc, Chicago, IL, USA). The data were expressed as percentage (%) and mean ± SD. Comparison of the means between the ALD patients and healthy volunteers was performed by Chi-square test. p > 0.05 was taken to be statistically significant.
RESULTS
The findings of the TB, DB, AST, ALT, ALP, GGT, and GSH levels among alcoholic and nonalcoholic groups are represented in Table 1. It was observed that the mean serum levels of total (2.9 ± 1.5 mg/dL) and direct bilirubin (1.6 ± 1.0 mg/dL) were significantly higher in patients with ALD than the controls. Similarly, the activities of enzymes such as AST (68.7 ± 1.629 IU/L), ALT (29.8 ± 6.5IU/L), ALP (1.4 ± 3.4 IU/L), and GGT (1.18 ± 8.4 mg/dL) were seen to increase significantly in the ALD patients than the healthy controls (p > 0.05), whereas the concentration of GSH was significantly lower in alcoholic patients (46 ± 6.8 mg/dL) (p > 0.05).
Aspartate transaminase/alanine transaminase ratio has been useful in differentiating the cause of liver damage in medical diagnosis of the disease.14,15 We observed an increase in the AST/ALT ratio (>2, 2.3) in alcoholic patients which was not statistically significant (p %3C; 0.05) (Table 2).
According to the age group, of 50 patients with ALD, the highest number was seen between the age group of 36–46 years with 44% followed by 28% in 25–35 years of age. Whereas in between 47 and 57 years of age, 24% patients had ALD and the remaining 4% was found in between the age group of 58–68 years (Fig. 1).
Sikkim is categorized by multiple ethnic groups such as Bhutia, Lepcha, Kiratis (Rai, Limboo, Tamang, Gurung, Mangar), and Gorkhas (Chettri and Bahun) and the alchohol consumption has been customarily dominant in the state. In our study, the Kiratis were found to be the highest suffering from ALD comprising 40% (n = 20) of the population. On the other hand, 26% (n = 13) were Gorkhas and Bhutias with 24% (n = 12), whereas the remaining 5% were the Lepchas (Table 3).
| Parameters | Alcoholics (M ± SD) | Nonalcoholics (M ± SD) |
|---|---|---|
| TB (mg/dL) | 2.9 ± 1.5 | 0.71 ± 0.24 |
| DB (mg/dL) | 1.6 ± 1.0 | 0.20 ± 0.04 |
| AST (IU/L) | 68.7 ± 1.6 | 40.8 ± 5.4 |
| ALT (IU/L) | 29.8 ± 6.5 | 36.2 ± 7.1 |
| ALP (IU/L) | 1.4 ± 3.4 | 1.75 ± 5.7 |
| GGT (mg/dL) | 1.18 ± 8.4 | 36.9 ± 9.7 |
| GSH (mg/dL) | 46 ± 6.8 | 61.5 ± 2.4 |
| Enzymes | Alcoholics group | Controlled group | p value |
|---|---|---|---|
| AST/ALT ratio | %3E;2 (2.3) | <2 (1.1) | 0.112** |
*p > 0.05 is significant, **p < 0.05 is insignificant
DISCUSSION
In the present study, the biochemical markers were compared between patients with ALD and normal healthy volunteers. Alcohol ingestion leads to a number of cellular changes as well as to the oxidant–antioxidant system.16,17 We observed a significant increase in the direct and total bilirubin level in alcoholic patients on comparison with healthy groups which is in consort with many studies.18,19 The activity of the enzymes such as ALT, AST, ALP and GGT was also found to increase, and similar results have been reported lately as well as in the past.19–21 Among the enzymes studied, GGT is the best indicator of excessive alcohol consumption.19 The increase in the enzyme activity may be due to the oxidative stress causing an injury to the hepatocytes or due to the accumulation of the metabolites induced from ethanol metabolism,22 which is evident from our study where the concentration of the GSH was decreased leading to the generation of reactive oxygen species. The findings have been supported by Subir et al.23 Though these aminotransferases (AST, ALT) are the sensitive markers of hepatocyte damage but they are not an ideal marker alone.24 The increase of theAST/ALT ratio (>2) may be due to the deficiency of pyridoxine-5-PO4 which then reduces the activity of ALT leading to the release of mitochondrial AST in serum.25
The age at which people initiates drinking has been declined with the development of economy and an improvement in the living standard.19 According to the National Family Health Survey-4 from 2015 to 2016, alcohol consumption between 15 years and 49 years of age was found to be 52.9%.5 We observed the highest number of alcoholic patients (25%) between the age group of 36–46 years and lowest between the age group of 58–68 years (4%).
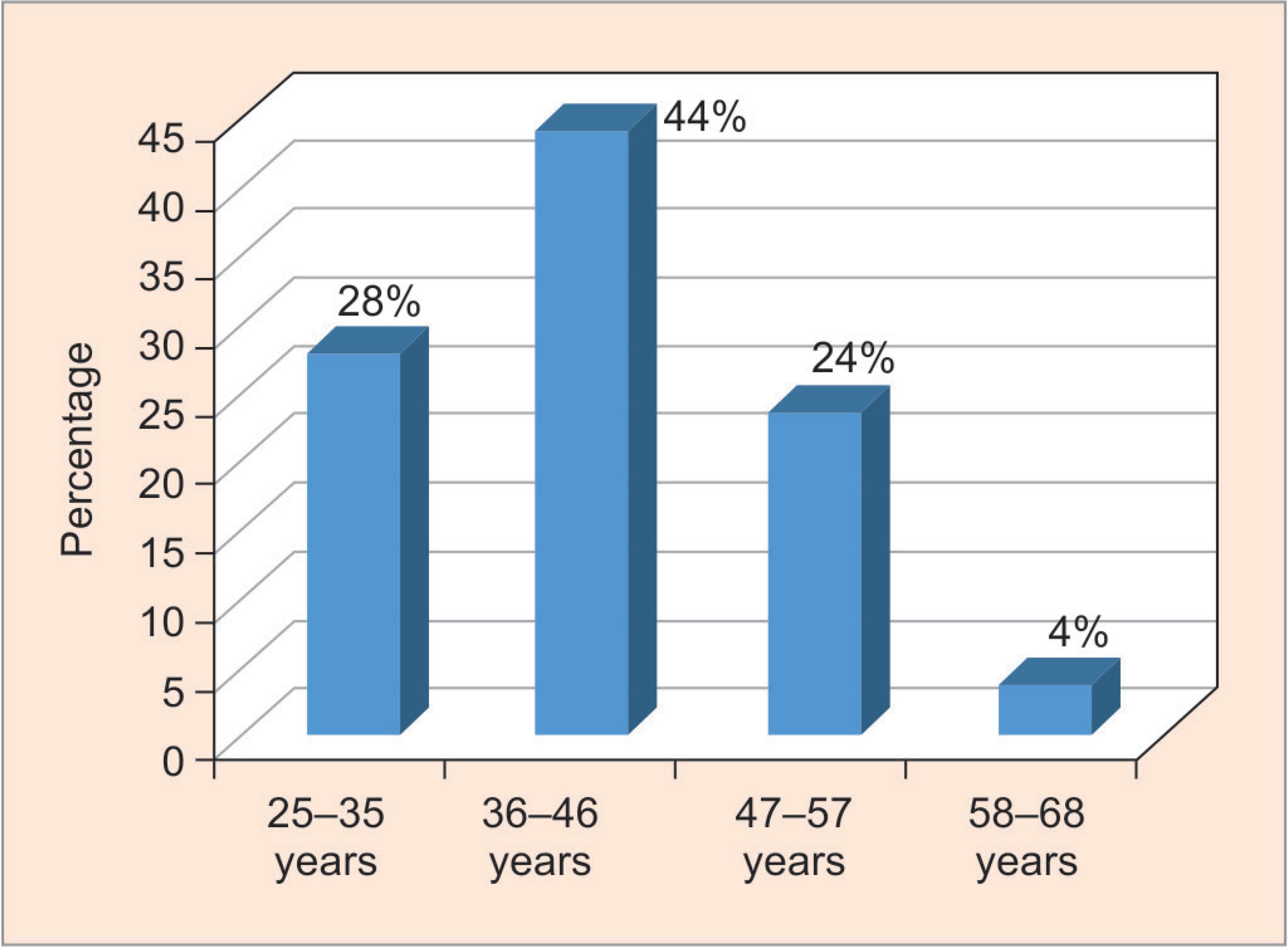
Fig. 1: Frequency distributions of patients with alcoholic liver diseases according to different age groups
| Ethnicity | No. of patients | Percentage |
|---|---|---|
| Bhutia | 12 | 24 |
| Lepcha | 5 | 10 |
| Kiratis | 20 | 40 |
| Gorkhas | 13 | 26 |
| Total | 50 | 100 |
Alcohol consumption is influenced by factors such as social and cultural norms, ethnicity, and geographical locations. Among the ethnic groups of the state, Kiratis were found to be the highest suffering from ALD (40%) and the Lepchas (5%) being the lowest. This distribution is aligned to the population distribution proportion of Kiratis in Sikkim.
CONCLUSION
Our study suggests a high number of ALD patients among Kiratis and in between the age group of 36–46 years. The oxidative stress markers were significantly altered among patients with alcoholic liver disease when compared with nonalcoholics. Further randomized studies need to be conducted to understand the relationship of biology of redox status among ALD with different population and its association with sociocultural practices and an influence of geographical locations.
ETHICAL APPROVAL
The article does not contain any studies with animals performed by any contributors.
Since the study is a part of the M. Sc dissertation work so ethical approval is not mandatory in our institute.
INFORMED CONSENT
Informed consent was obtained from all participants included in the study.
REFERENCES
1. Jaurigue MM, Cappell MS. Therapy for alcoholic liver disease. World J Gastroenterol 2014;20(9):2143–2158. DOI: 10.3748/wjg.v20.i9.2143.
2. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease: AASLD Practice Guidelines. Hepatology 2010;51(1):307–328. DOI: 10.1002/hep.23258.
3. Tsukamoto H. Conceptual importance of identifying alcoholic liver disease as a lifestyle disease. J Gastroenterol 2007;42(8):603–609. DOI: 10.1007/s00535-007-2075-3.
4. National Institute of Alcohol Abuse and Alcoholism, U.S Department of Health and Human Services, The Physicians’ guide to helping patients with alcohol problems. Washington, DC: Government Printing Office; 1995.
5. National Family Health Survey-4, 2015–2016. Ministry of Health and Family Welfare, Government of India. International Institute of Population Sciences,Mumbai.
6. Hagymasi K, Blazovics A, Lengyel G, et al. Oxidative damage in alcoholic liver disease. Eur J Gastroenterol Hepatol 2001;13(1):49–53. DOI: 10.1097/00042737-200101000-00009.
7. Sathian B, Sreedharan J, Baboo NS, et al. Relevance of sample size determination in medical research. Nepal J Epidemiol 2010;1(1):9–10.
8. Jendrassik L, Grof P. Colorimetric method of determination of bilirubin. Biochem Z 1938;297:81–82.
9. Thefelt W, Hoffmeister H, Busch EW, et al. Reference values for the determination of GOT, GPT, and alkaline phosphatase in serum with optimal standard methods. Dtsch Med Wschr 1974;99(8):343.
10. Bergmeyer HU, Scheive P, Wahlefeld AW. Clin Chem 1978;24:58.
11. Bergmeyer HU, Horder M. IFCC methods for the measurement of catalytic concentrations of enzymes. part 3, IFCC. Method for alanine aminotransferase (l-alanine 2 -oxoglutarate aminotransferase, ec 2.6.1.2) Clin Chem Acta 1980;105(1):147–172. DOI: 10.1016/0009-8981(80)90105-9.
12. Szasz GA. Kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem 1969 Feb;15(2):124–136.
13. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J lab Clin Med 1963;61:882–888.
14. Nyblom H, Berggren U, Balldin J, et al. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2000;39(4):336–339. DOI: 10.1093/alcalc/agh074.
15. Nyblom H, Björnsson E, Simrén M, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int September 2006;26(7):840–845. DOI: 10.1111/j.1478-3231.2006.01304.x.
16. Gopal DV, Rosen HR. Abnormal findings on liver function tests. Interpreting results to narrow the diagnosis and establish a prognosis. Postgrad Med 2000;107(2):100–102. DOI: 10.3810/pgm.2000.02.869 ,105–109, 113–114.
17. Desai M. Eco System and Ethnic Constellation of Sikkim. Calcutta: Netaji Institute of Asian Studies; 1988. p. 39.
18. Das SK, Nayak P, Vasudevan DM. Biochemical Markers for Alcohol Consumption. Ind J Clin Biochem 2003;18(2):111–118. DOI: 10.1007/BF02867376.
19. Torkadi PP, Apte IC, Bhute AK. Biochemical Evaluation of Patients with Alcoholic Liver Disease and Non-alcoholic Liver Disease. IJCB 2014;29(1):79–83. DOI: 10.1007/s12291-013-0310-7.
20. Moulali D. A Study on Gamma Glutamyl Transferase and Amylase Levels in Chronic Alcoholics with Hepatitis. IJSRP 2015;5(11):758–764.
21. Correia JP, Alves PS, Camilo EA. SGOT-SGPT ratios. Dig Dis Sci 1981;26(3):284. DOI: 10.1007/BF01391646.
22. Pradhan R, Lekharu R, Srivastava R, et al. A Study of Oxidative Stress in Alcoholic Liver Disease. GCSMC J Med Sci 2014;III(I):16–17.
23. Das SK, Vasudeva DM. Biochemical diagnosis of alcoholism. IJCB 2005;20(1):35–42. DOI: 10.1007/BF02893039.
24. Das SK, Vasudevan DM. Monitoring oxidative stress in patients with alcoholic and nonalcoholic liver disease. IJCB 2005;20(2):24–28. DOI: 10.1007/BF02867396.
25. Gupta S, Pandey R, Katyal R, et al. Lipid peroxide levels and antioxidant status in alcoholic liver disease. Indian J Clin Biochem 2005;20(1):67–67. DOI: 10.1007/BF02893045.
________________________
© The Author(s). 2019 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.