REVIEW ARTICLE | https://doi.org/10.5005/jp-journals-10054-0191 |
RAGE and Serum Copeptin Act as a Potential Biomarker for Chronic Kidney Disease with and without Type 2 Diabetes Mellitus
1,2,5Department of Biochemistry, Integral Institute of Medical Sciences and Research, Integral University, Lucknow, Uttar Pradesh, India
3Department of Biochemistry, King George’s Medical University, Lucknow, Uttar Pradesh, India
4Department of Medicine, Integral Institute of Medical Sciences and Research, Integral University, Lucknow, Uttar Pradesh, India
6Department of Basic Medical Sciences, Integral Institute of Allied Health Sciences and Research, Integral University, Lucknow, Uttar Pradesh, India
Corresponding Author: Roshan Alam, Department of Biochemistry, Integral Institute of Medical Sciences and Research, Integral University, Lucknow, Uttar Pradesh, India, Phone: +91 8340131885, e-mail: drroshan@iul.ac.in
How to cite this article Mishra D, Alam R, Ahmed MK, et al. RAGE and Serum Copeptin Act as a Potential Biomarker for Chronic Kidney Disease with and without Type 2 Diabetes Mellitus. Indian J Med Biochem 2021;25(3):131–134.
Source of support: Self-funded and supported by Institute
Conflict of interest: None
ABSTRACT
The critical role of receptors for advanced glycation end products (RAGEs) and serum copeptin in the progression of chronic diseases and their complications has recently become more apparent. This review summarizes the recent contributions to the field of RAGEs and serum copeptin in chronic kidney disease (CKD). Over the past 2 decades, RAGEs have been seen to be involved in the progression of CKD, and specifically, it leads to diabetic nephropathy. Although several in vitro and in vivo studies highlighten the detrimental role of AGEs accumulation in tissue injury. Recent studies have focused on the novel mechanisms that contribute to end-organ injury as a result of AGEs accumulation, as well as novel targets of therapy in kidney disease. Serum copeptin is related to the severity of the disease in autosomal dominant polycystic kidney disease (ADPKD), and it helps in the prediction of future renal events (decline in renal function and increase in total kidney volume). The main purpose of this review is to evaluate critically the role of RAGE and serum copeptin as a prognostic biomarker for renal outcomes in ADPKD, and it will be potentially helpful as a predictive marker of treatment response. As the prevalence and the incidence of CKD rises all over the world, nowadays it is very essential to identify the therapeutic strategies which must either delay the progression and aggressiveness of CKD or to improve the mortality rate in this population. The main focus of this review is to highlight the recent studies that enhance our current understanding of the mechanisms that mediate AGEs-induced progression in CKD as well as novel treatment strategies that have the potential to avoid this disease process. This review presents current knowledge regarding the roles of RAGE and copeptin in CKD with and without diabetes mellitus. Studies from human subjects are presented to highlight the breadth of evidence linking RAGE and copeptin to CKD consequences of these metabolic disorders.
Keywords: Adipocytes, Advanced glycation end product, Amadori products, Aminoguanidine, Autosomal dominant polycystic kidney disease, Chronic kidney disease, Copeptin, Diabetes mellitus, Nephropathy, Receptors for advanced glycation end products, Schiff base.
INTRODUCTION
Chronic kidney disease (CKD) is a condition associated with progressive loss of kidney function and kidney damage. The two common causes of CKD are diabetes mellitus (DM) and hypertension. Other causes of CKD also include polycystic kidney disease, obstructive uropathy, and primary glomerulonephritis.1 Chronic kidney disease patients present elevated advanced glycation end products (AGEs) blood levels. Advanced glycation end products promote inflammation through binding to their receptor (RAGE), located on the membrane of mesangial cells, endothelial cells, and macrophages.2 Chronic kidney disease patients have many affected physiological pathways. Variations in the genes regulating these pathways might affect the incidence and predisposition to this disease. Chronic kidney disease patients are also characterized by high genomic instability. This instability could be translated to high levels of genetic damage measured by the incidence of chromosomal damage (micronuclei) when their cells are challenged with ionizing radiation and could be either the cause or the consequence of renal pathologies. In addition, it has been observed that CKD patients repair less efficiently DNA damage.3 Diabetes mellitus is a disorder of multiple etiologies, where alteration is characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion, insulin action or both.4 The chronic hyperglycemia of diabetes is associated with a significant long-term squeal, particularly damage and/or dysfunction and failure of various organs, especially the kidneys, eyes, nerves, heart, and blood vessels.5 Diabetic nephropathy is one of the main microvascular complications of DM and is a leading cause of end-stage renal disease (ESRD). It is widely accepted that diabetic nephropathy is a heterogeneous disorder caused by the interaction between environmental and genetic factors.6 We have seen the discovery of the receptor for AGE products (RAGE; The name for its gene is AGER) in 1992 on account of this molecule’s ability to bind the products of non-enzymatic glycation and oxidation of proteins/lipids, the advanced glycation end products, or AGEs.6 Advanced glycation end products are not the only biomarkers of a hyperglycemic and pro-inflammatory/pro-oxidative state; rather they also play a very important role in the pathogenesis of complications associated with DM, in the larger part through their interactions with RAGE. Various AGEs are also generated in highly heated and processed foods.7 Hence, the interaction of AGE with RAGE shows both from endogenously-formed AGE adducts and from dietary AGE sources. RAGE is expressed on multiple types of cells, such as vascular cells, immune cells, neurons, cardiomyocytes, adipocytes, glomerular epithelial cells or podocytes, lung epithelial cells, and a wide range of transformed cells, both in animal models and human subjects.8–10
Formation of AGEs
Refer to Flowchart 1.
Receptors for Advanced Glycation End Products (Rage)
When AGEs interact with their cellular receptors, it plays an important role in the pathogenesis of diabetic complications.11 Receptors for advanced glycated end products have been recognized as a receptor for amyloid-beta peptide (Aβ) and β-sheet fibrils [65] S100/calgranulins;12 amphoterin13 and Mac-1.14 RAGE is made up of three extracellular domains, which contains a V-type that contains ligand binding properties and two C-type immunoglobulin domains C 1, and C 2, a transmembrane helix, and a short cytosolic tail.15 A fourth transmembrane domain anchors RAGE in the membrane and is connected to a highly charged fifth intracellular domain that mediates interaction with cytosolic transduction molecules. RAGE acts as a signal transduction receptor for N3-carboxymethyl-lysine (CML), the major AGE in vivo, and is likely to interact with other AGEs.16 Advanced glycation end products bind only to the V domain of RAGE (Flowchart 2).16,17
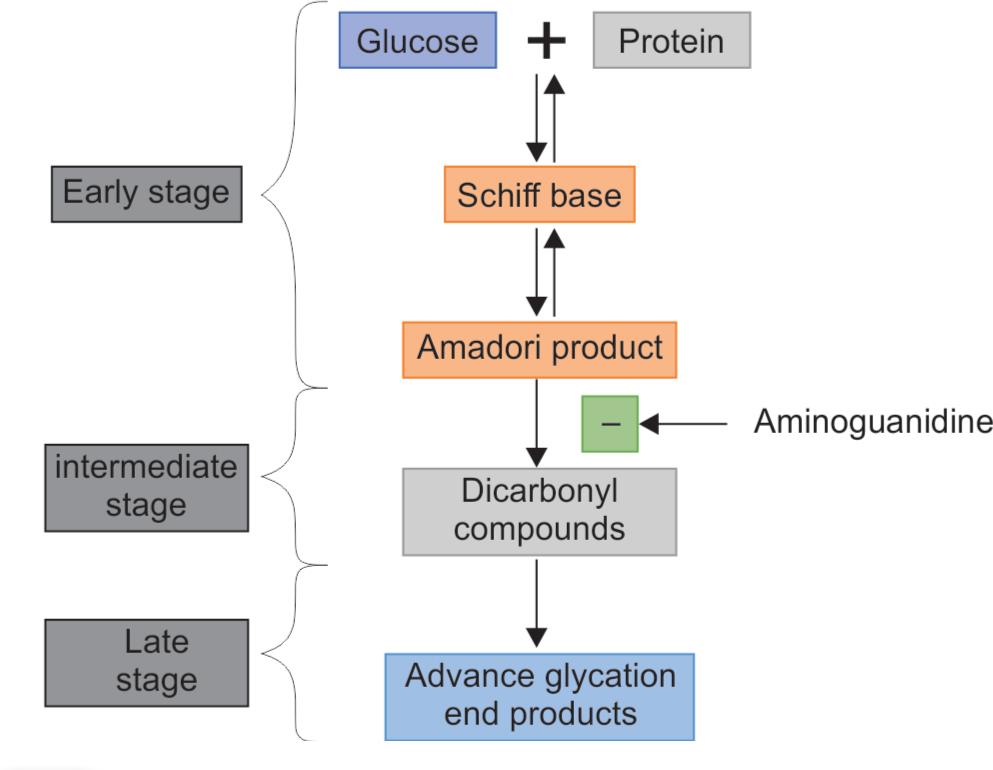
Flowchart 1: Formation of advanced glycation end products in three stages, i.e., early, intermediate, and late-stage involving (AGEs). In an early stage, sugars react with a free amino group to form a Schiff base which undergoes a rearrangement to a more stable product known as the Amadori product. In an intermediate stage, the Amadori product degrades to a variety of reactive dicarbonyl compounds. In the late stage of the glycation process, AGEs (irreversible compounds) are formed
Ages and Diabetic Complications
- Diabetic retinopathy.
- Diabetic cataract.
- Diabetic nephropathy.
- Diabetic neuropathy.
- Diabetic cardiomyopathy.
Diabetic nephropathy is defined as a progressive decline in glomerular filtration rate, accompanied by proteinuria and other end-organ complications such as retinopathy.18 A renal disease that occurs in patients suffering from DM is characterized by hemodynamic (hyperfiltration and hyperperfusion) as well as there are structural abnormalities (glomerulosclerosis, alterations in tubulointerstitium including interstitial fibrosis) and metabolic changes also take place.19 It has been reported that the AGE-RAGE axis plays a very important role in diabetic nephropathy.
Arginine vasopressin (AVP) is an important pituitary hormone in humans that mediates osmoregulation, water balance, and tonicity. Disturbances in AVP regulation contribute to the pathogenesis of diabetes insipidus and cardiovascular diseases (CVDs).14,20,21 However, the measurement of endogenous plasma AVP level is difficult due to technical limitations and is not widely available. Copeptin, a 39 glycosylated amino acid, is the carboxyl-terminal part of precursor-AVP and is released from the hypothalamus synergistically with AVP. It is easier to measure copeptin rather than AVP in serum due to its superior stability. Previous studies have demonstrated a high functional correlation between copeptin and mature AVP supporting it as an appropriate surrogate marker instead of AVP.22 Copeptin is partly cleared by the kidneys and hence, the level of copeptin is higher in CKD or ESRD patients, compared to those with preserved kidney function.13,23 Associations between copeptin and the development of heart failure, myocardial infarction, diabetes insipidus, hyponatremia, DM, and metabolic syndrome have been reported. The importance of copeptin in kidney diseases has also been illustrated in the recent literature. In those with ESRD and type 2 DM, a higher copeptin level was associated with an increased risk of stroke, sudden cardiac death, and all-cause mortality.12 An elevated copeptin level was noted to be associated with reduced estimated glomerular filtration rate (eGFR) and simple renal cyst growth in patients with autosomal dominant polycystic kidney disease (ADPKD).24,25 Therefore, it is proposed that copeptin can serve as a marker of the progression of the renal cystic disease (Flowchart 3).
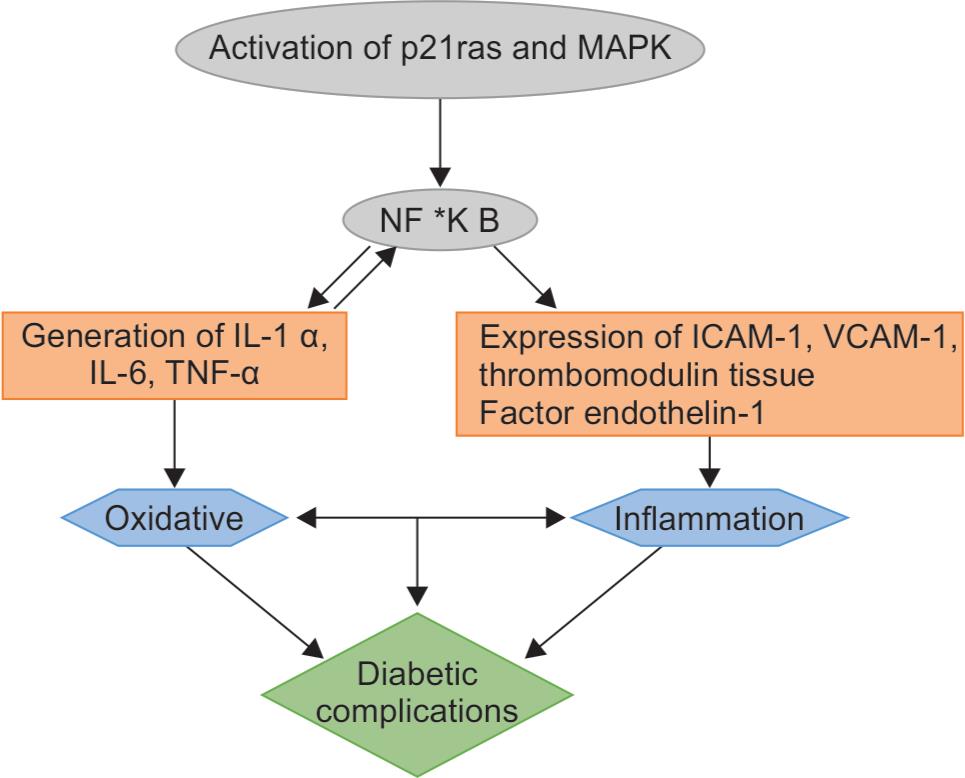
Flowchart 2: Interaction of AGE with RAGE which leads to oxidative stress and initiation of inflammation cascade takes place which involves the activation of MAPK pathway, NF-kB, IL-6, TNF-α, expression of ICAM-1 and VCAM-2 which ultimately leads to diabetic complications
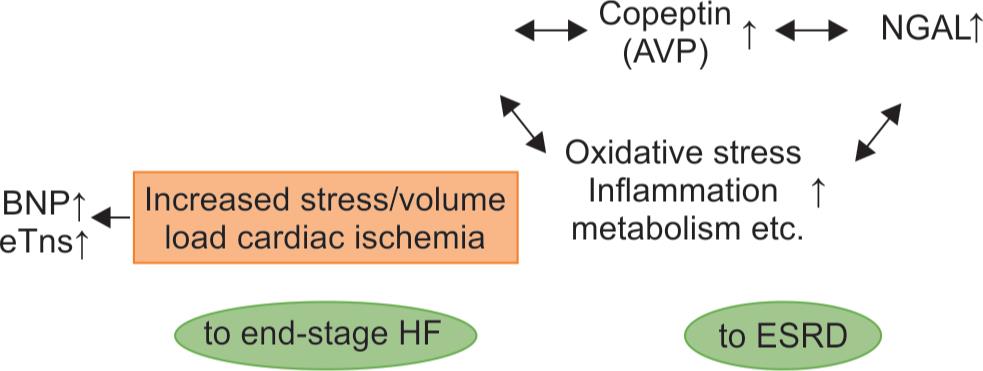
Flowchart 3: Interrelationships and pivotal roles of the five emerging biomarkers in cardiorenal syndrome. AVP, arginine vasopressin; BNPs, B-type natriuretic peptides (BNP and NT-proBNP), CKD, chronic kidney disease; cTns, cardiac troponins (troponin I and T); CVD, cardiovascular disease; ESRD, end-stage renal disease; FGF-23, fibroblast growth factor-23; and NGAL, neutrophil gelatinase-associated lipocalin
Cardiovascular disease is closely associated with CKD and ESRD and is well shown as the leading cause of morbidity and mortality in the patients with CKD, most notably those on ESRD or dialysis.20,26 The prevalence of concomitant coronary artery disease (CAD), left ventricular hypertrophy (LVH), congestive heart failure (CHF), cardiac arrhythmia (most commonly atrial fibrillation), and valvular/vascular calcification are increased in CKD patients. Recently, the term, cardiorenal syndrome (CRS) has been introduced in an attempt to emphasize the interaction between the CV and renal systems in acute or chronic disease settings.21,27
CONCLUSION
The two common causes of CKD are DM and hypertension. Other causes of CKD also include polycystic kidney disease, obstructive uropathy, and primary glomerulonephritis. The receptor for advanced glycation end-products (RAGE) is a multi-ligand cell surface receptor of the immunoglobulin superfamily and it has been associated with kidney disease in both non-diabetic and diabetic patients. Presently, data on the association between RAGE and CKD while numerous studies have reported associations of RAGE with diabetic complications in other populations. The present study aims to explore the possibility of using RAGE as candidate markers of CKD in patients with DM. Previous studies have suggested that chronically high vasopressin is deleterious to renal function. Here, we evaluated the association of plasma copeptin, a surrogate of vasopressin, with the incidence of CKD in the general population. High copeptin levels are associated with the development and the progression of CKD in the general population. Copeptin has turned out to give valuable prognostic information for future cardiovascular events. However, since its plasma concentration directly depends on renal function, the value of copeptin as a predictor for outcome also in patients with CKD is unknown. Plasma content of copeptin increases with the advancement of CKD. The purpose of this study was to evaluate copeptin content as a potential marker of CKD. Plasma copeptin may help to identify subjects with diabetic CKD who are at high risk for renal function decline. If with the help of the present study the diagnosis and prognosis is verified then accordingly the management of the case could be done. This study will help in reducing the mortality rate which occurs due to CKD. Early determination of the complications and management of the cases could be done. Adds to the growing body of literature about the utility of RAGE and copeptin as a marker to portend adverse outcomes. Further studies should confirm these findings along with addressing the limitations of this report. In addition, larger studies examining whether the increased formation of advanced glycated end products and lowering copeptin in CKD could help us answer if the observed relationship between copeptin and decline in kidney function is causally related.
REFERENCES
1. Wong FN, Chua KH, Kuppusamy UR, et al. Association of the receptor for advanced glycation end-products (RAGE) gene polymorphisms in Malaysian patients with chronic kidney disease. PeerJ 2016;4:e190. DOI: 10.7717/peerj.1908.
2. Baragetti I, Norata GD, Sarcina C, et al. -374 T/A RAGE polymorphism is associated with chronic kidney dProgression in subjects affected by nephrocardiovascular sisease. PLoS ONE 2013;8(4):e60089. DOI: 10.1371/journal.pone.00600896.
3. Corridor Z, da Silva Filho MI, Rodríguez-Ribera L, et al. Genetic variants associated with chronic kidney disease in a Spanish population. Sci Rep 2020;10(1):144. DOI: 10.1038/s41598-019-56695-2.
4. Nayak BS, Roberts L. Relationship between inflammatory markers, metabolic and anthropometric variables in the Caribbean type-2 diabetic patients with and without microvascular complications. J Inflamm 2006;17(3):1–7.
5. Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem 1992;267(21):14987–14997. DOI: 10.1016/S0021-9258(18)42137-0.
6. Inan-Eroglu E, Ayaz A, Buyuktuncer Z. Formation of advanced glycation endproducts in foods during cooking process and underlying mechanisms: a comprehensive review of experimental studies. Nutr Res Rev 2019;2019(1):1–13. DOI: 10.1017/S0954422419000209.
7. Shekhtman A, Ramasamy R, Schmidt AM. Glycation and the RAGE axis: targeting signal transduction through DIAPH1. Expert Rev Proteom 2017;14(2):147–156. DOI: 10.1080/14789450.2017.1271719.
8. López-Díez R, Shekhtman A, Ramasamy R, et al. Cellular mechanisms and consequences of glycation in atherosclerosis and obesity. Biochim Biophys Acta 2016;1862(12):2244–2252. DOI: 10.1016/j.bbadis.2016.05.005.
9. Ramasamy R, Shekhtman A, Schmidt AM. The multiple faces of RAGE–opportunities for therapeutic intervention in aging and chronic disease. Expert Opin Therapeut Targets 2016;20(4):431–446. DOI: 10.1517/14728222.2016.1111873.
10. Roussel R, Fezeu L, Marre M, et al. Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab 2014;99(12):4656–4663. DOI: 10.1210/jc.2014-2295.
11. Han D, Yamamoto Y, Munesue S, et al. Induction of receptor for advanced glycation end products by insufficient leptin action triggers pancreatic β-cell failure in type 2 diabetes. Genes Cells 2013;18(4):302–314. DOI: 10.1111/gtc.12036.
12. Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. DOI: 10.1038/35012626.
13. Chavakis T, Bierhaus A, Al-Fakhri N, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med 2003;198(10):1507–1515. DOI: 10.1084/jem.20030800.
14. Stern DM, Yan SD, Yan SF, et al. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 2002;1(1):1–15. DOI: 10.1016/s0047-6374(01)00366-9.
15. Kislinger T, Fu C, Huber B, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 1999;274(44):31740–31749. DOI: 10.1074/jbc.274.44.31740.
16. Xie J, Reverdatto S, Frolov A, et al. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J Biol Chem 2008;283(40):27255–27269. DOI: 10.1074/jbc.M801622200.
17. O’Connor AS, Schelling JR. Diabetes and the kidney. Am J Kidney Dis 2005;46(4):766–773. DOI: 10.1053/j.ajkd.2005.05.032.
18. Bohlender JM, Franke S, Stein G, et al. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289(4):F645–F659. DOI: 10.1152/ajprenal.00398.2004.
19. Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from kidney disease: improving global outcomes (KDIGO). Kidney Int 2011;80(6):572–586. DOI: 10.1038/ki.2011.223.
20. Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010;31(6):703–711. DOI: 10.1093/eurheartj/ehp507.
21. Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 2013;9(4):223–239. DOI: 10.1038/nrneph.2013.22.
22. Morgenthaler NG, Struck J, Jochberger S, et al. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab 2008;19(2):43–49. DOI: 10.1016/j.tem.2007.11.001.
23. Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/ calgranulin polypeptides. Cell. 1999;97(7):889–901. DOI: 10.1016/s0092-8674(00)80801-6.
24. Fenske W, Wanner C, Allolio B, et al. Copeptin levels associate with cardiovascular events in patients with ESRD and type 2 diabetes mellitus. J AM Soc Nephrol 2011;22(4):782–790. DOI: 10.1681/ASN.2010070691.
25. Yan J, Navaneethan SD. Copeptin and decline in kidney function. Am J Nephrol 2016;44(1):19–21. DOI: 10.1159/000447369.
26. Tasevska I, Enhörning S, Persson M, et al. Increased levels of copeptin,a surrogate marker of AVP, are associated with an increased risk of CKD in a healthy population. Am J Nephrol 2016;44(1):22–28. DOI: 10.1159/000447522.
27. Velho G, El Boustany R, Lefèvre G, et al. Plasma copeptin, kidney outcomes, ischemic heart disease, and all-cause mortality in people with long-standing type 1 Diabetes. Diabetes Care 2016;39(12):2288–2295.
________________________
© The Author(s). 2021 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.